Clinical Decision Support
Medical Device grade apps and models in Gynecological Ultrasound and Early Pregnancy Care.
Decision support applications
Clinical decision support
Gynaia develops software tools that enable accurate, standardized, and reproducible decision-making in gynecological ultrasound.
Grounded in
real world data
Gynaia's apps implement rigorously validated models from IOTA, IETA, & other research studies and peer reviewed work, supporting routine use with confidence.
Accessible and professional
All apps run on smartphones and the web, designed for certified professionals in gynecology, oncology, obstetrics, fertility, and early pregnancy care.
Professional apps
Gynaia develops and delivers software tools that support accurate, standardized, and reproducible clinical decisions in gynecological ultrasound.
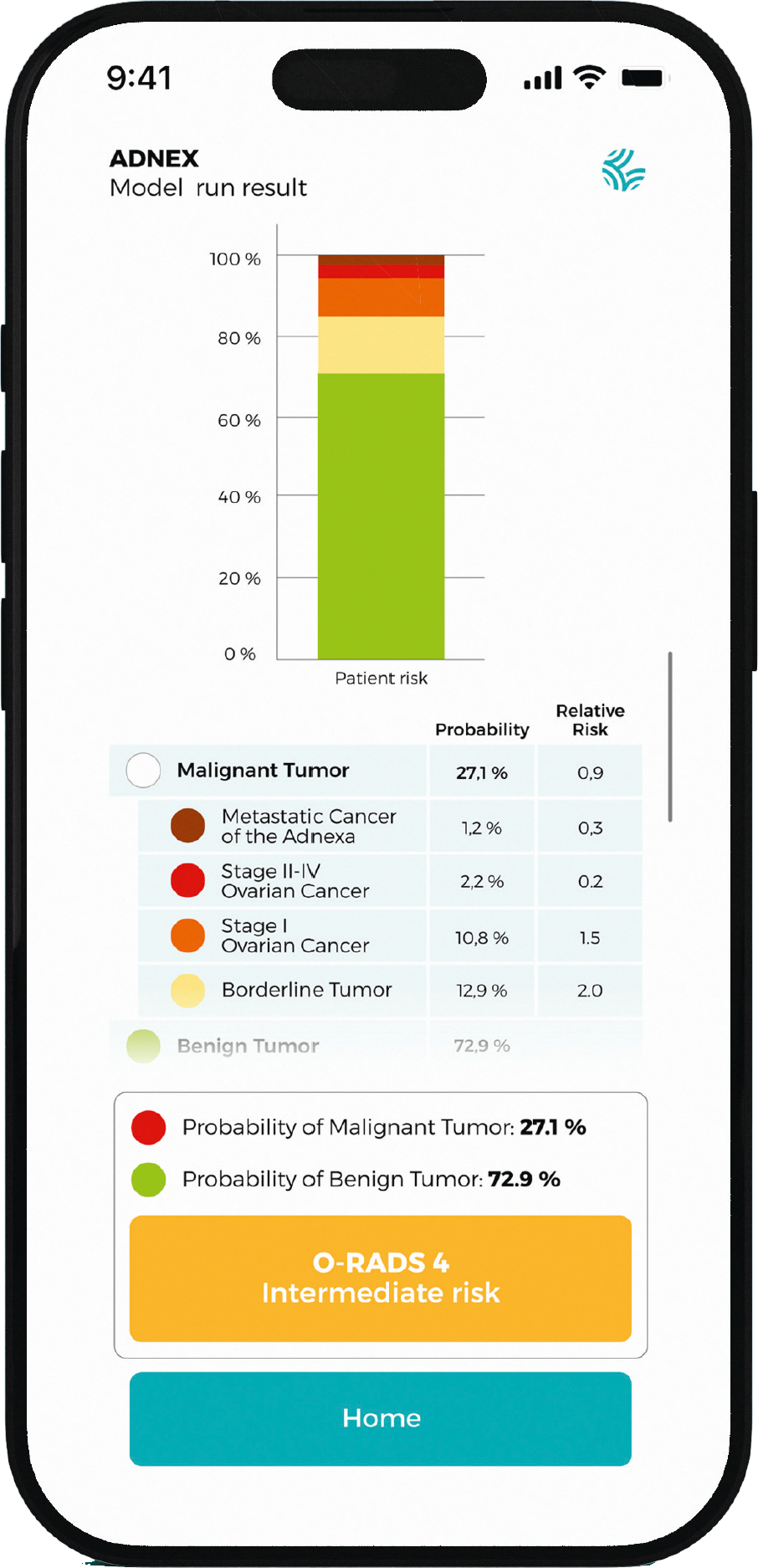
Classifying Adnexal Masses with Confidence
Developed by the IOTA consortium, the ADNEX app supports clinicians in estimating the risk that an adnexal mass is benign or malignant - and distinguishes between subtypes of ovarian tumors.
The latest version, ADNEX2, was announced at ISUOG 2025 and improves diagnostic performance, including for conservatively managed cases.
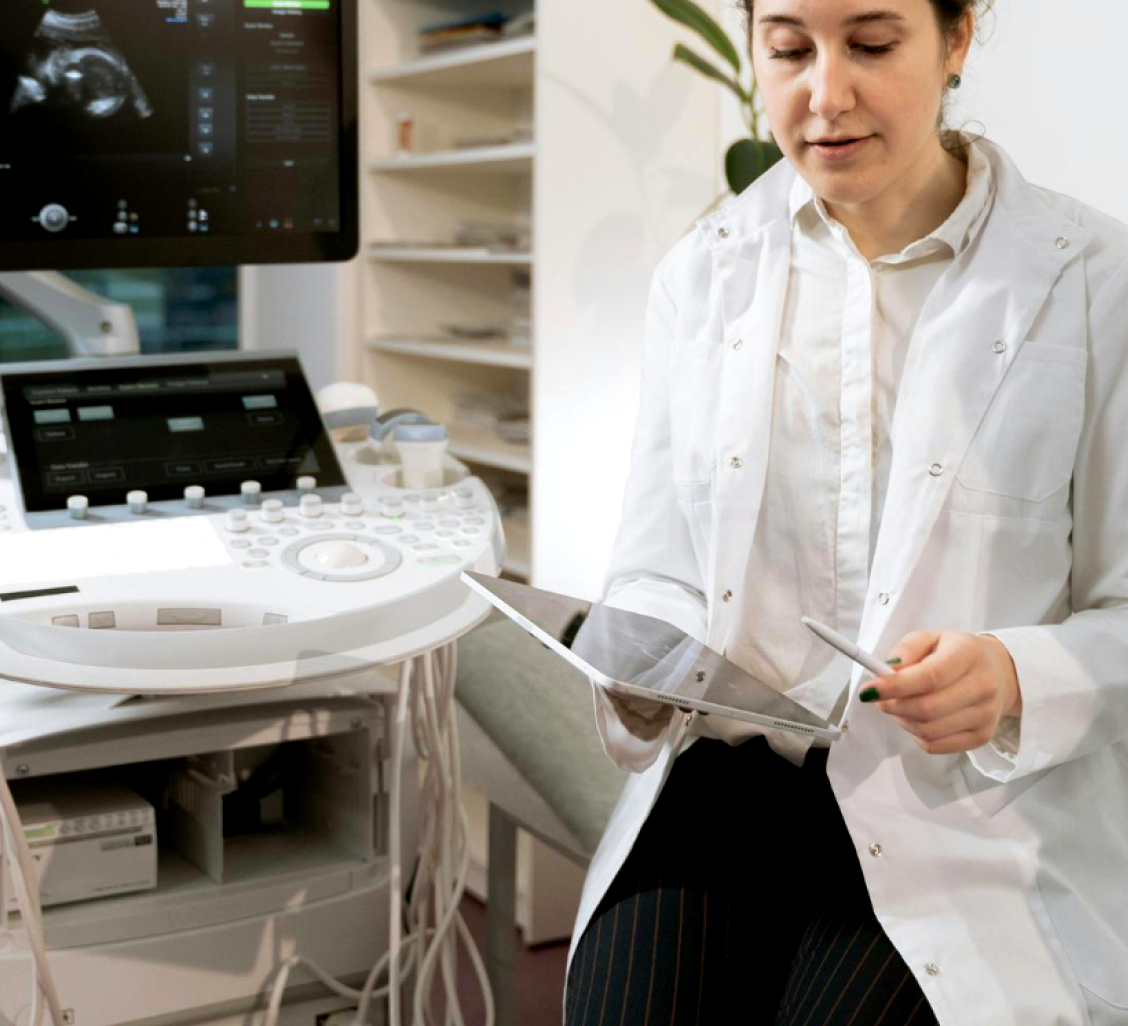
Endometrial App
(IETA Model)
This upcoming application will assist in evaluating endometrial abnormalities, including intracavity lesions and suspected endometrial cancer. It incorporates the IETA terminology and scoring system, with structured input fields and contextual guidance to support consistent reporting.
- Based on the IETA framework (International Endometrial Tumor Analysis)
- Designed for endometrial thickness assessment, vascularity scoring, and lesion characterization
- Integrates structured reporting elements and terminology
- Suitable for use in routine practice in select areas with regulatory approval
Early Pregnancy Decision Support
Gynaia's early pregnancy tool combines two validated models to support clinical decision-making in challenging early pregnancy scenarios:
M6/PUL Model:
Estimates the risk of ectopic pregnancy in women with a pregnancy of unknown location (PUL) using clinical data (age, pain, bleeding, history of ectopic pregnancy) and biochemical markers (β-hCG and progesterone). Helps triage patients into low- or high-risk categories and guides appropriate follow-up.
PUV Model:
Assesses the likelihood of viability in intrauterine pregnancies of uncertain viability (PUV) based on clinical features (age, bleeding, gestational age) and ultrasound findings (gestational sac size and yolk sac presence).
Validation studies have shown high discriminatory performance across large, prospective cohorts.
Get Started with Gynaia
Improve patient outcomes with learning & development, quality assessment, and clinical decision support.